Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
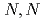 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E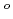 ,
, 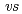 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 93(12), p.767 - 770, 2005/12
Times Cited Count:10 Percentile:56.74(Chemistry, Inorganic & Nuclear)Electrochemical and spectroelectrochemical has been used to investigate the behaviour of Neptunium (VI) in concentration 1-8 M HNO solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E
solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E ) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO
) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO
solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO
solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO solution.
solution.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
JAERI-Conf 2005-007, p.341 - 344, 2005/08
Electrochemistry has been used to investigate the behavior of plutonium(IV) in 1-7 M HNO solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E
solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E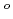 ) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO
) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO
solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO solution.
solution.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Radiochimica Acta, 93(2), p.75 - 81, 2005/02
Times Cited Count:9 Percentile:53.19(Chemistry, Inorganic & Nuclear)no abstracts in English
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 262(2), p.311 - 315, 2004/11
Times Cited Count:11 Percentile:58.51(Chemistry, Analytical)no abstracts in English
; ; ; Tachikawa, Enzo
Denki Kagaku, 54, p.974 - 976, 1986/00
no abstracts in English